years of existence
+1000 projects completed
+15 clinical diagnostic areas
50 collaborators
Cerba Xpert supports you in the IVDR steps




An end-to-end solutions thanks to our belonging
to the Cerba Healthcare group, offering easy patients recruitment

A team of 50 experts
With a team of highly skilled professionals including: Project managers, CRAs, Lab technicians, Data Analysts, Biobank specialists, and Quality & Regulatory affairs experts, Cerba Xpert is committed to delivering high-quality, reliable, and timely solutions
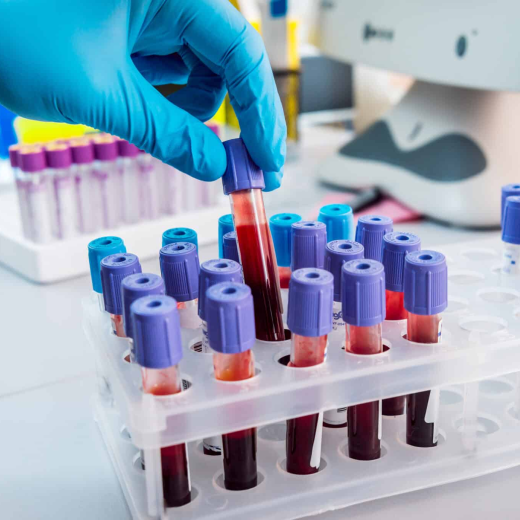
A complete offer with our Biobank 40k+ samples
Access to European, African & American samples including blood and blood derivatives (whole blood, plasma, serum), various biofluids (cerebrospinal fluid, urine, bone marrow, saliva), and other sample formats (FFPE tissues, stools, swabs, and microbiology isolates).

Cerba Xpert belongs to Cerba Healthcare group
Cerba Healthcare is an international medical diagnostics laboraty who covers all spheres of medical biology: Routine & specialized clinical pathology, anatomical pathology, and is present on all continents. This integration allows us to provide our clients with a comprehensive offer.
Why choose Cerba Xpert ?
From study design to the Final Report through regulatory submissions, study setup, patient recruitment, study monitoring, and statistical analysis, Cerba Xpert provides a full suite of services tailored to your needs.
Cerba Xpert ensures compliance with the stringent requirements of the EU IVDR 2017/746, helping you navigate the complex regulatory landscape smoothly.
Cerba Xpert adheres to ISO 9001 and Good Clinical & Lab Practices rigorous quality management systems, ensuring the highest standards of data integrity and participant safety.


